Thermal Infrared Spectrum of Earth From Space
| Home | Energy Physics | Nuclear Power | Electricity | Climate Change | Lighting Control | Contacts | Links |
|---|
DEFINITION:
Global warming is the increase in average dry bulb temperature caused by the combination of the transient increase in the rate of solar energy absorption caused by melting of ice and the transient decrease in the rate of infrared radiation emission caused by increases in Green House Gas (GHG) concentrations in Earth's atmosphere.
HISTORY:
The visible light emitted by the sun can be separated into a spectrum of colors (frequencies) using a diffraction grating. Close examination of the solar spectrum shows a number of dark spectral lines. Each dark line corresponds to a frequency at which the sun's outer atmosphere is opaque (does not transmit light). These narrow dark lines arise because certain gases in the sun's outer atmosphere randomly scatter photons of certain frequencies.
During the 1960s various space craft sent to the planet Venus reported that the surface temperature of Venus was far hotter than previously believed and that Venus atmosphere was primarily composed of carbon dioxide (CO2) and methane (CH4).
The reason for Venus high surface temperature is that CO2 and CH4 are opaque in part of the thermal infra red emission spectrum.
The surface temperature issues on Earth, known as the Greenhouse Effect and Global Warming, arise because Earth's atmosphere is also opaque in certain infrared frequency bands. This issue is obvious in the far infrared thermal emission spectrum of the Earth as viewed from outer space.
THE GREENHOUSE EFFECT:
The Greenhouse Effect causes Earth's cloud temperature to be warmer than it would be if the planetary Bond albedo remained the same but Green House Gases (GHGs) were not present in the Earth's atmosphere. The Green House Effect is primarily caused by carbon dioxide, water vapor, ozone, methane and certain other trace gases in the Earth's upper atmosphere.
What happens is as follows:
1. Normally about 30% of the solar radiation power incident on the Earth reflects off the Earth into outer space, unchanged in frequency. The remaining 70% of the incident solar radiation is absorbed as heat by either the atmosphere, dry land or the ocean.
2. At steady state, the law of conservation of energy requires that the average flow of infrared radiant energy emitted by Earth into space equals the average flow of solar energy absorbed by Earth. However, due to the presence of CO2 and water vapor in the Earth's upper atmosphere, in certain infrared frequency bands the Earth's upper atmosphere is opaque. In these frequency bands infrared radiation is emitted into space not from clouds, but from points higher in the Earth's upper atmosphere which are much colder.
3. The rate of infrared energy emission decreases rapidly with decreasing temperature. Hence the infrared radiation emission rate is much less in frequency bands where high altitude greenhouse gases make the atmosphere opaque.
However, at steady state conservation of energy requires that the average infrared radiation emission rate must equal the average solar radiation absorption rate. Addition of more greenhouse gas molecules to the upper atmosphere causes it to become more opaque to infra red radiation emitted from below. As the infrared radiation emission rate into outer space is reduced in some frequency bands, in other frequency bands where the atmosphere is transparent the infrared radiation emission rate must increase. The only way for that infrared radiation emission rate to increase is for the temperature at the the point of emission (cloud tops) to increase.
4. The Greenhouse Effect is complicated by temperature related changes in local Bond albedo. As the local cloud top emission temperature rises through 273.15 K in the presence of water there is a step decrease in local albedo from as high as 0.50 to as low as 0.035.
BACKGROUND:
Earth is constantly absorbing a fraction of the incident solar radiation from the sun. Earth is constantly emitting infrared radiation into outer space. Historically, over the hundreds of thousands of years prior to the industrial revolution, Earth reached a steady state average temperature distribution at which the average rate of solar energy absorption approximately equalled the average rate of infrared energy emission. During the 800,000 years immediately preceeding the industrial revolution the atmospheric CO2 concentration remained in the range 180 ppmv to 300 ppmv. Just prior to the industrial revolution the atmospheric carbon dioxide (CO2) concentration was about 280 parts per million by volume (ppmv).
At this initial steady state condition there were two other important balances:
a) The rate at which CO2 went into solution in near polar ocean waters approximately equalled the rate at which CO2 came out of solution in tropical ocean waters;
b) The rate of injection of CO2 into the ocean-atmosphere pool approximately equalled the rate of removal of CO2 from the ocean-atmosphere pool.
During the atmospheric nuclear bomb tests of the 1950s to 1960s, by measurement of the atmospheric C-14 concentration versus time, it was determined that the CO2 in the atmosphere reaches equilibrium with the CO2 in ocean solution with an exponential equilibrium time constant of about 16 years. Hence for times long compared to 16 years the CO2 in the atmosphere and oceans can be viewed as a single ocean-atmosphere pool.
Since WWII man has been burning fossil fuels at a prodigious and increasing rate. The resulting injection of fossil CO2 into Earth's atmosphere has raised the atmospheric CO2 concentration which has significantly changed Earth's infrared radiation emission spectrum. The nearly simultaneous absorption of CO2 by the oceans has significantly increased the ocean (HCO3)- ion concentration.
Combustion of fossil fuels also produces fine soot particles. The soot particles deposit on snow and ice surfaces causing increased solar energy absorption and melting which decreases Earth's solar reflectivity.
EARTH'S INFRARED EMISSION:
Infrared emission is reduced by an increase in atmospheric Green House Gas (GHG) concentration. Carbon dioxide (CO2), water vapor (H2O) and methane (CH4) are all GHGs. The recent increase in CO2 concentration is a result of combustion of fossil fuels. The increase in atmospheric water vapor concentration is a result of increasing ocean and ground surface temperature.
On November 24, 1996 a US interplanetary spacecraft known as the Mars Global Surveyor recorded the far infrared radiation spectrum emitted by Earth.

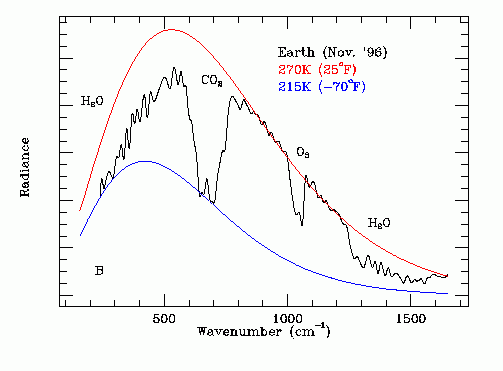
Thermal Infrared Spectrum of Earth From Space
The black line on this graph shows the experimentally measured amplitude (Np Ep) of the Earth's infrared emission spectrum as a function of:
Wave Number = (Fp / C),
as recorded from deep space where:
C = speed of light = 3.0 X 10^10 cm / s
Note the combined influence of high altitude CO2, lower altitude water vapor and medium altitude ozone on this spectrum. These GHGs reduce the infra red emission into outer space.
Note that the underlying black body shape of this spectrum indicates a lower altitude emission temperature at the cloud tops of 270 K.
Note that the dominant source of the emitted infrared radiation is lower altitude water molecules transitioning from the liquid phase to the solid phase at 273.15 K. The photon energy at the center frequency of the radiation is the heat of fusion per molecule of water.
Note that at frequencies within the CO2 absorption band thermal radiative emission from the cloud tops to outer space is obscured and thermal emission occurs at a higher altitude at temperature of about 215 K. The CO2 in the upper atmosphere is at 215 K, a much lower temperature than the freezing water in the lower atmosphere. For frequencies within the CO2 absorption band the higher altitude CO2 absorbs radially propagating 273 deg K photons emitted by freezing water in the lower atmosphere and then emits omni-directional photons, half of which propagate into outer space and half of which propagate back down toward Earth.
Note that this process also has the effect of increasing the apparent width of the CO2 infrared absorption band as viewed from outer space. The consequent global warming effect of CO2 is much greater than indicated by simple laboratory CO2 infrared absorption measurements made with uniform pressure and temperature CO2.
Atmospheric ozone behaves in an analogous manner to CO2.
THE REALITY OF GLOBAL WARMING:
1) Measurements of atmospheric CO2 concentration at Mona Loa indicate that from the commencement of the industrial revolution to 1957 the atmospheric CO2 concentration rose 35 ppmv from about 280 ppmv to 315 ppmv. From 1957 to 2024 the atmospheric CO2 concentration rose 107 ppmV from 315 ppmV to 422 ppmV. Today the atmospheric CO2 concentration is rising at about 2.5 ppmv / year.
2) By 1979 preliminary satellite measurements of Earth's infrared thermal emission spectrum indicated that doubling of the atmospheric CO2 concentration would result in about a 3 degree C dry bulb temperature increase at temperate and tropical latitudes due to IR effects alone.
3) If the present trend in fossil fuel use continues it is reasonable to project an atmospheric CO2 concentration of over 560 ppmv by the year 2100.
4) In the case of communities in northern Canada, due in part to a change in average local albedo, the corresponding average dry bulb temperature increase is projected to be about 9 degrees C.
THE RADIANT POWER BALANCE:
Planet Earth is surrounded by a vacuum. The only way for Earth to gain significant heat is by absorption of solar radiation from the sun. The only way for Earth to lose significant heat is by emission of thermal infrared radiation (IR) to outer space. Hence the Law of Conservation of Energy gives:
[Rate of net heat absorption] =
[Rate of solar radiation absorption] – [Rate of IR radiation emission]
Earth's surface temperature is the result of a balance between absorbed radiant solar power and emitted thermal infrared radiation (IR) that has lasted for more than 800,000 years. During that period on average Earth was neither absorbing nor emitting net heat energy.
Then the Law of Conservation of Energy gives for the historical reference period:
[Rate of net heat absorption] =
[Rate of solar radiation absorption] – [Rate of IR radiation emission]
= 0
Thus during the historical reference period:
[Solar radiation absorption] =[IR radiation emission]
IR RADIATION EMISSION:
A spectrometer is an instrument that displays the power conveyed by each frequency component of electromagnetic radiation. A spectrometer indicates radiation power per unit frequency increment as a function of frequency. A spectrometer outputs a graph with units of Power / Hz on the vertical axis and Hz on the horizontal axis.
The area under the resulting spectral curve is the total thermal power conveyed by the measured electromagnetic radiation.
On November 24, 1996 a US interplanetary spacecraft named the Mars Global Surveyor, while on its way to Mars, looked back at Earth against a dark space background and recorded the infrared emission spectrum shown by the black line on the following graph:

Thermal Infrared Spectrum of Earth From Space
On this graph:
Radiance = Relative Photon Energy Flux Per Unit Frequency
and
Wavenumber = [Photon Frequency] / [Speed of Light C]
Note that the radiant power per unit frequency goes to zero at both high and low frequencies. That spectral feature keeps the total area under the black line finite and hence keeps the total radiant power finite.
At any instant in time the area under the black line is Earth's total IR Radiant Emission power.
Note the various notches in the black line caused by different Green House Gases (GHGs).
EARTH'S SOLAR RADIATION ABSORPTION:
The fraction of incident solar radiation reflected off planet Earth is Fr, where Fr is known as the planetary Bond albedo. The fraction Fr is a strong function of the fraction of Earth's surface that is covered by white cloud (microscopic ice particles in clouds) or by surface ice. The presence or absence of ice is determined by whether or not the local temperature of the relevant H2O molecules is above or below 273.15 degrees K. Visible light photographs of Earth taken from outer space show that the local solar reflectivity is low (~ 0.10) near Earth's equator and is high (~ 0.50) near Earth's poles. The average solar reflectivity (planetary Bond albedo) measured in 1999-2000 was about 0.297 +/- 0.005. Melting of ice decreases Fr and hence increases the rate of solar energy absorption. The fraction of incident solar radiation absorbed by Earth is:
(1.00 - Fr)
The decrease in the local value of Fr with decreasing latitude and hence with increasing atmospheric temperature is clearly shown on the following photograph.

Earth From Space Apollo 17 Dec. 1972
This photo clearly shows that as the future atmospheric temperature near Earth's poles approaches the present atmospheric temperature near Earth's equator Earth's average solar reflectivity will decrease from about 0.30 to about 0.10, which when Earth reaches steady state will cause a lower atmosphere infrared emission temperature increase, as seen by an observer in outer space, of about 17.5 degrees C.
A complicating issue is that the local solar reflectivity (local Bond albedo) has a large step discontnuity at a local emission temperature of 273.15 degrees K, the freezing point of water. This large step discontinuity gives Earth's atmosphere multiple semi-stable operating states.
In the "cool" state condensed water in clouds is ice and the local Bond albedo (local solar reflectivity) is about 0.50. In the "hot" state condensed water in clouds is liquid or vapor with a much smaller local Bond albedo. In the "hot" state in the tropics the average local Bond albedo (local solar reflectivity) is about 0.10.
In 2018 Earth's atmosphere was in the "warm" state in the tropics and was in the "cool" state in near polar regions. In 1999-2000 using moonshine Earth's average solar reflectivity (planetary Bond albedo) was measured to be about 0.29.
THERMAL ENERGY ABSORPTION:
When there is a change in parameters which causes the absorbed solar power to exceed the emitted infrared power there is net energy accumulation by Earth.
Over dry land this energy accumulation heats the surface soil, which promptly rises in temperature. This increase in soil temperature promptly increases thermal infrared radiation emission. Thus the net energy absorption by dry land soon stops, with the land at a slightly increased temperature.
Over the open ocean the net energy accumulation heats water to a depth of about 200 feet. The resulting change in ocean surface temperature occurs veery slowly and hence takes a very long time to affect thermal infrared emission from the ocean surface. However, the warmer water floats on top of deeper colder water. Due to wind and ocean currents the warm water spreads over the ocean surface and eventually reaches and melts polar floating ice. This melting of polar floating ice causes a large decrease in the planetary reflectivity (planetary Bond albedo) which in turn causes a large increase in the absorbed solar power.
As long as there is floating polar ice it takes decades for an increase in net heat absorption to noticeably increase ocean temperature which increases the IR emission from the ocean surface.
The consequential increase in net absorbed thermal power will eventually raise the ocean surface temperature which in turn will increase the wet bulb temperature and will decrease the cloud solar reflectivity over the ocean. Ultimately that decrease in solar reflectivity will cause an extinction level temperature rise.
STEP RESPONSE:
Over thousands of years of stable atmospheric gas concentrations a balance was established between absorbed solar power and emitted infrared power.
Assume that today there is a sudden step increase in a GHG concentration. Further assume that during the measurement period the
[Rate of solar radiation absorption]
remains constant. Then at the instant of the step increase in atmospheric GHG concentration the area under the black line decreases due to an increase in the area of the respective GHG notch.
Hence the Law of Conservation of Energy gives:
[Rate of net heat absorption] =
[Rate of solar radiation absorption] – [Rate of IR radiation emission]
where now the
[Rate of net heat absorption]
by planet Earth is positive due to the drop in
[Rate of IR radiation emission].
After some time the ongoing net heat absorption will cause Earth's surface temperature to rise. Due to the behavior of thermal radiation the rise in Earth surface temperature will cause the black line to shift slightly upwards and to the right until the area under the black line is again equal to the [solar radiation absorption]. However, now the GHG notch is bigger than it was originally.
Note that the effect of a sudden step increase in atmospheric GHG is to temporarily reduce the area under the black line by enlarging the GHG notches. At the instant the atmospheric concentration of greenhouse gases increases the notch area increases causing the total area under the black line to decrease.
Subsequent to the step increase in GHG concentration Earth's surface will gradually absorb net heat until the resulting increase in Earth surface temperature causes Earth's [IR radiation emission] to again equal the [solar radiation absorption].
The net absorbed heat promptly raises the surface temperature of dry ground, but, due to the ocean's higher heat capacity, raises the ocean surface temperature only very slowly.
From a human observer's perspective the dry ground surface temperature is the dry bulb temperature whereas the ocean surface temperature is the wet bulb temperature.
In summary, a step increase in atmospheric GHG concentration causes a prompt increase in dry bulb temperature and a lagging increase in wet bulb temperature.
SOLAR RADIATION ABSORPTION:
Most of Canada has seasonal snow and ice cover. Even a small average air temperature rise due to IR effects significantly reduces the average annual snow and ice cover and hence increases
[net heat absorbtion]
because the solar reflectivity (albedo) of snow and ice is typically about 3X the solar reflectivity of dry ground and is about 10X the solar reflectivity of open water.
Recall that the Law of Conservation of Energy gives:
[Rate of net heat absorption] =
[Rate of solar radiation absorption] – [Rate of IR radiation emission]
Note that an increase in
[Rate of solar radiation absorption]
increases the
[Rate of net heat absorption]
COMBINED EFFECTS:
Thus, in circumpolar countries there is increased transient net heat absorption due to decreases in average albedo and due to increased GHG gas concentrations in Earth's atmosphere. The increased net heat absorption causes a prompt increase in dry bulb temperature and a lagging increase in wet bulb temperature.
In northern Canada these two effects combine to produce an overall average dry bulb temperature increase over dry land of about 3X that in tropical countries.
However, there is a time lag of years between the increase in average dry bulb temperature over land and the corresponding increase in ocean temperature and hence wet bulb temperature. This time lag may be decades when the initial conditions involve floating sea ice.
A consequence of this time lag is that even if all fossil fuel combustion is stopped tomorrow the wet bulb temperature will continue increasing for many subsequent years.
Hence the world has yet to experience the full consequences of the GHGs that have already been injected into the atmosphere.
THE WET BULB TEMPERATURE PROBLEM:
Over the ocean, when the solar radiation exceeds the IR emission, due to ocean surface turbulence it takes years for the average ocean surface temperature to rise in response.
However, that rise in ocean surface temperature determines the ultimate average rise in wet bulb temperature, which is a critical parameter for those who live in the tropics.
The wet bulb temperature is the temperature at which there is no net evaporation or condensation of water because the surrounding air is saturated with humidity. This temperature is crucial in the tropics because as the wet bulb temperature rises towards the human body temperature the human body can no longer cool itself by evaporation of perspiration.
In summary, a step rise in atmospheric CO2 concentration produces a corresponding prompt rise in dry bulb temperature and a lagging rise in wet bulb temperature, where that lag may be years.
Hence, even if we could stop emission of fossil CO2 tomorrow the wet bulb temperature would continue to rise for years into the future.
However, the actual atmospheric situation is much worse than set out above because there is no capability of quickly stopping fossil CO2 emissions. Stopping fossil CO2 emissions requires sufficient sources of replacement economic, clean and dependable (non-fossil) power to displace existing combustion of fossil fuels and requires an effective mechanism for preventing further extraction of fossil fuels.
MITIGATING GLOBAL WARMING:
Some GHGs will naturally decompose over time. However, significantly reducing the atmospheric CO2 concentration requires so much energy as to be considered practically impossible. What can be done is to prevent further fossil CO2 injection into the atmosphere. That
requires economic, sustainable and dependable sources of clean energy sufficient to displace the thermal power presently provided by combustion of fossil fuels.
Presently fossil fuels provide a dependable thermal power of about 20,000 GWt. By comparison all of the world's presently operating nuclear reactors provide a thermal power of only about 1400 GWt.
Worse yet, with a handful of exceptions all of these are water cooled thermal neutron reactors that rely on the rare uranium isotope U-235 for fuel, which is in short supply around the world. There is not enough economic U-235 to fuel existing and planned thermal neutron reactors for their projected working lives.
To mitigate climate change it is necessary to build a new fleet of fuel sustainable fast neutron reactors that do not rely on U-235. These reactors must convert abundant fertile atoms into fissile atoms and then fission the fissile atoms. These reactors are capacity limited by their inventory of TRU. TRU are atoms with atomic numbers greater than 92 that can be extracted from used CANDU fuel or that can be made using a process known as neutron spallation.
Displacing fossil fuels will require construction of thousands of fuel sustainable Fast Neutron Reactors (FNRs), of which only a handful exist today.
Hence, even if maximum available resources are applied to solving this problem, the climate situation will continue getting worse for several decades into the future due to continuing fossil CO2 emission.
TECHNICAL EVOLUTION:
During WWII the allies acting together built ocean going ships at the rate of more than three a day and four engine bombers at more than three an hour. The rate at which 300 MWe FNRs must be built to arrest CO2 driven climate change is about one a day for 45 years.
To make this FNR solution happen CNS members and their supporters must overcome the incompetence and corruption within our present governments and their agencies.
Unless CNS members can make the voters believe that widespread deployment of fuel sustainable fast neutron reactors is the only solution to climate change, then they are all doomed to be near term victims of climate change.
In 1965 Canada had the industrial capacity to make neutron spallation equipment.
In the 1960s we knew that thermal neutron reactors had a limited future energy output capacity due to their dependence on the rare uranium isotope U-235.
In 1970 the atmospheric CO2 concentration data from Mona Loa indicated that within my lifetime the atmospheric CO2 concentration would become a major problem. Nevertheless Ontario Hydro and other major electricity utilities proceeded with construction of massive coal fired electricity generation.
By 1979 we knew from satellite data that doubling the atmospheric CO2 concentration would raise the dry bulb temperature by about 3 degrees C.
In 1990 Canada was still making large CANDU reactors that can be used to supply TRU for Fast Neutron Reactors.
In 1994 the USA had the industrial capacity to make Fast Neutron Reactors.
By 1994 we knew how to build Fast Neutron Reactors (FNRs) to solve the CO2 problem, but at that time North American politicians were enamored with fossil fuels.
In a series of ill thought governmental actions the organizations harboring these skill sets were destroyed. If young people today want to live, they are going to have to acquire these skill sets. Today these skill sets mostly reside in persons who are more than 75 years old.
In the 2000s the fossil fuel industry promoted intermittent renewable electricity generation because every kWh of wind electrity needed at least 2 kWh of fossil fuel balancing capacity.
Wind turbines and solar panels are also required to assist in making TRU for the aforementioned FNRs.
Today the only way to mitigate climate change is to leave fossil fuels in the ground and live with the attendant consequences, one of which is reliance on Fast Neutron Reactors for dependable power.
Electricity grids must be continuously energized by dependable power sources. Today the only source of dependable clean electricity that can sustainably displace fossil fuels is Fast Neutron Reactors (FNRs).
*******************************************************
CONCISE EXPLANATION OF GLOBAL WARMING:
1. The law of conservation of energy requires that:
(Net heat flux absorption by the Earth)
= (Solar radiant energy absorbed) - (infrared radiant energy emitted)
2. Prior to the industrial revolution the Earth was at a steady state condition where the average emission temperature was constant implying that:
(Solar radiant energy absorption) = (infrared radiant energy emission)
3. Experimental measurements since 1978 have shown that:
(Incident solar radiant energy per unit time) = constant +/- 0.3%.
4. About 1905 Albert Einstein discovered that:
a) Radiant energy is absorbed or emitted in energy packets known as photons.
b) Each photon has a characteristic frequency Fp.
c) The energy Ep carried by a particular photon is given by:
Ep = h Fp
where:
h = Planck constant
5. Let Np = number of infrared photons emitted by the Earth per unit time per unit frequency
Then the total number of infrared photons emitted per unit time is given by:
Integral from Fp = 0 to Fp = infinity of:
Np dFp
6. The radiant energy emitted by the Earth per unit time is given by:
Integral from Fp = 0 to Fp = infinity of:
Np Ep dFp
where:
Np Ep is the amplitude of the infrared spectrum at frequency Fp.
7. The black line on the following graph shows the experimentally measured amplitude (Np Ep) of the Earth's infrared emission spectrum as a function of:
Wave Number = (Fp / C),
as recorded from deep space by the Mars Global Surveyor space probe on November 24, 1996, where:
C = speed of light = 3.0 X 10^10 cm / s

8. The area under the experimentally measured black line is:
(total rate of infrared energy emission by the Earth) / C
9.Note the big notch in the center of the graph which is due to the opaqueness of the Earth's upper atmosphere at thermal infrared frequencies corresponding to photon scattering by carbon dioxide (CO2). In the middle of this opaque band the effective temperature at which the net infrared emission takes place is only 215 degrees K (- 58 degrees C) so Np falls by about 50%.
10. As the concentration of CO2 in the atmosphere increases the CO2 related notch in the graph becomes wider and deeper and hence the area under the black line decreases. Hence the infrared energy emission by the Earth in the notch frequency band decreases.
11. Since the rate of solar energy supply remains constant the net energy absorption by the Earth increases. This net energy absorption becomes heat which causes melting of ice and ocean warming.
12. Water vapor (H2O) behaves in a manner analogous to CO2. Warming the ocean surface increases the concentration of atmospheric H2O, which further reduces the area under the black line on the above data graph and hence further reduces infrared emission into outer space.
13. After the polar ice has melted the average ocean temperature will gradually increase which will cause the black line on the above graph on the right hand side of the CO2 notch to gradually move upwards to make the total thermal infrared power emission equal the total solar power absorption as required by the law of conservation of energy. The upward movement of the black line causes an upward movement of the red line, which corresponds to an increase in the planetary emission temperature.
*****************************************************************
COMPLICATIONS RELATING TO GLOBAL WARMING
STEP DISCONTINUITY:
A complicating issue is that the local solar reflectivity (local Bond albedo) has a large step discontnuity at a local emission temperature of 273.15 degrees K, the freezing point of water. This large step discontinuity gives Earth's atmosphere multiple semi-stable operating states.
In the "cool" state condensed water in clouds is ice and the local Bond albedo (local solar reflectivity) is about 0.50. In the "hot" state condensed water in clouds is liquid or vapor with a much smaller local Bond albedo. In the "hot" state in the tropics the average local Bond albedo (local solar reflectivity) is about 0.10.
Today in 2024 Earth's atmosphere is in the "warm" state in the tropics and is in the "cool" state in near polar regions. Today Earth's average solar reflectivity (planetary Bond albedo) is below 0.29 and the atmospheric CO2 concentration is about 422 ppmv.
SPECTRAL INTERACTION OF GREENHOUSE GASES
Carbon dioxide alone does not cause a lot of global warming. Water vapor alone does not cause a lot of global warming. These gases have complimentary spectral windows which limit the amount of warming when these gases are only individually present. However, when these gases are present simultaneously, each gas partially blocks the thermal infrared transmission spectral window of the other gas, causing much more warming than the simple sum of the two individual gas warming contributions. Thus in the presence of water vapor the impact of raising the atmospheric CO2 concentration is more than would be the case were the water vapor not present. Similarly, the impact of raising the atmospheric water vapor concentration is more in the presence of CO2 than would otherwise be the case if the CO2 were not present. These two effects feed back on each other. Global warming deniers generally either overlook or do not understand this spectral issue.
METHANE MISCONCEPTION:
A common misconception is related to methane and CO2. Methane alone is a strong infrared absorber on a per molecule basis. However, that absorption power has only minor direct practical effect in the Earth's atmosphere because the dominant methane absorpion band lies at the high frequency edge of the thermal infrared emission spectrum. However, over time (~ 10 years) methane spontaneously oxidizes to form more CO2, which increases the atmospheric CO2 concentration. Thus the rapid increase in the atmospheric CO2 concentration after a bulk methane release is indeed a potential threat to man kind. Large amounts of methane are trapped in methane hydrate (a muskeg and sea floor ice lattice known as cathrate). A geophysical event that triggers rapid melting of the methane hydrate can cause a large methane release to the atmosphere which over the subsequent decade becomes a large CO2 injection.
MELTING OF LAND BORNE GLACIERS:
The net radiant energy absorption causes land borne glaciers to melt and causes thermal expansion of the oceans. These two effects, if they are permitted to continue, will eventually cause an average sea level rise of about 80 m. A further problem is that in some places annual glacier melt water provides critical agricultural crop irrigation in the dry season. When the glaciers melt away, melt water is no longer available for agricultural crop irrigation in the dry season.
Viewed another way, due to increasing atmospheric CO2 concentration a lot of people in semi-arid countries and island states that presently have barely sufficient fresh water for agricultural irrigation will in the future starve to death due to insufficient fresh water for irrigation in the dry season. This process is already causing uncontrolled human migration out of parts of Africa.
OCEAN RELEASE OF CO2:
An imminent danger to our grand children is that heating the cold ocean causes the ocean to release more CO2 just as heating a can of a cold soda drink causes it to release CO2. This released CO2 will further increase the atmospheric CO2 concentration and will make the CO2 notch in the above graph wider and deeper. This effect is known as a positive feedback process.
*************************************************
CIRCUMSTANCES LEADING TO THERMAL RUNAWAY:
EFFECT OF WATER VAPOR:
The immediate effect of adding CO2 and other Green House Gases (GHGs) in the atmosphere is to cause net planetary heat absorption. In 1996 the average emission temperature outside the GHG absorption bands was measured to be about 270 degrees K (- 3.15 degrees C) and the corresponding Earth emissivity was about 0.80. The direct effect of doubling of the atmospheric CO2 concentration is to increase the average emission temperature by about 3.15 degrees C. However, when the emission temperature is increased so also is the atmospheric water vapor concentration. Water vapor is also a GHG. The increase in atmospheric water vapor concentration accompanying a doubling in atmospheric CO2 concentration further increases Earth's emission temperature by about 0.865 degrees C. Thus the total direct emission temperature increase caused by doubling the atmospheric CO2 concentration is:
3.15 C + 0.865 C = 4.015 C.
However, the direct increase in planetary emission temperature caused by increasing the atmospheric CO2 and H2O concentrations causes local heating which melts ice causing the latitude of the transition from the "warm" state to the "cool" state to increase. This increase in the transition latitude reduces the average solar reflectivity (planetary Bond albedo) of Earth. Hence Earth absorbs more solar radiation causing yet more planetary heating and hence causing the planetary emission temperature to further increase. This increase in planetary emission temperature causes yet more ice melting and causes the latitude of the "warm" state to "cool" state transition to further increase. Again Earth's solar reflectivity decreases and Earth's average emission temperature further increases. This process is assisted by ocean currents and wind that transport the extra absorbed heat from low latitude regions to high latitude regions.
If the atmospheric CO2 concentration exceeds a critical threshold Earth's entire atmosphere will progressively switch from the "cool" state to the "hot" state. This relatively rapid state change is known as thermal runaway.
The geologic isotope record shows that thermal runaway occurred on Earth 55 million years ago during a time period known as the Paleocene Eocene Thermal Maximum (PETM). During the PETM exposed organic fossil carbon burned, the polar ice caps completely melted and there was a global extinction of large land animals.
Thermal runaway is followed by a phenomena known as "warm state trapping". In the "cool" state cold open ocean water tends to absorb CO2 from the atmosphere. In the "hot" state warm open ocean water tends to emit CO2 to the atmosphere. However, if the whole ocean is in the "hot" state there is no path for atmospheric CO2 concentration reduction via ocean absorption of CO2. Hence when thermal runaway occurs Earth remains trapped in the "hot" state until biochemical processes which form fossil fuels and carbonate rock reduce the CO2 concentrations in the ocean and in the atmosphere sufficiently to enable a spontaneous "hot" state to "cool" state transition. During the PETM warm state trapping held Earth in the "hot" state for over 200,000 years.
Note that a local "cool" state to "warm" state transition occurs at a local emission temperature of 273.15 degrees K (0 degrees C). In 1996 Earth's average emission temperature was measured to be about 270 degrees K (- 3.15 C) indicating that tropical latitudes were already in the "warm" state and higher latitudes were still in the "cool" state. Since 1996 further injection of fossil CO2 into Earth's atmosphere has caused the averagee latitude of the "warm" state to "cool" state transition to increase. Local transition from the "cool" state to the "warm" state has caused large changes in local average temperatue in much of northern Canada.
A major concern is that doubling of the atmospheric CO2 concentration will increase the average Earth emission temperature from 270 K to 274 K at which point much of the water in the atmosphere no longer exists as ice. When ice micro-crystals in clouds melt the local Bond albedo sharply decreases causing local heat accumulation. Then wind and ocean current driven heat transport cause ice melting at high latitudes.
Another major concern is that the residency time of non-equilibrium CO2 in Earth's atmosphere is about 16 years and the residency time in the ocean-atmosphere pool is over 100,000 years. Hence, even if fossil fuel use is suddenly halted the heat absorption by the oceans due to the non-equilibrium atmospheric CO2 concentration will continue. This protracted ocean heating will melt all floating ice leading to a further decrease in planetary albedo, will release large quantities of methane trapped in permafrost and will result in further sea level rise due to ocean thermal expansion.
The geologic record shows that thermal runaway has occurred in the past. The only uncertainty is the exact excess atmospheric CO2 concentration required to trigger uncontrollable thermal runaway today. The longer that there is excess CO2 in the atmosphere the warmer the ocean surface will become leading to an ever higher latitude for the "warm" state to "cool" state transition and hence a greater propensity for uncontrollable thermal runaway
.
Note that if the atmospheric temperature at the altitude where water is located rises only 2.3 degrees K the solar reflectivity (planetary albedo) will rapidly fall causing the lower atmosphere temperature to rapidly rise.
At lower atmosphere temperatures significantly above 273 degrees K the IR absorption and emission by water molecules occurs as a result of a continuum of vapor-liquid molecular phase transitions. These transitions occur over a wide range of pressure dependent temperatures and hence are much more broad spectrum in nature than the liquid-solid phase transition which occurs at a single temperature which is almost independent of pressure.
As the lower atmosphere temperature increases so also does the atmospheric H2O concentration. A significant H2O molecule concentration in the middle atmosphere will reduce the IR emission into outer space at frequencies less than the CO2 absorption band frequency.
The aforementioned warming mechanisms form a high gain positive feedback loop at a local emission temperature of 273.15 degrees K. At low atmospheric CO2 concentrations the loop gain is less than unity. Presently on average the emission temperature is less than 273.15 K and the CO2 concentration is about 420 ppmv. Presently the atmosphere is unstable as is indicated by storms but over time these storms spontaneously decay. The storm violence is greater at lower latitudes where the ocean surface and atmosphere are warmer than at higher latitudes where the ocean surface and the atmosphere are cooler. As the atmospheric CO2 concentration continues to increase the thermal feedback loop gain will exceed unity, and a condition of rapid net spontaneous heat absorption known as thermal runaway will occur. Thermal runaway will cause melting of all ice on Earth and over time will cause an average lower atmosphere temperature rise of more than 17.5 degrees C.
TRIP THRESHOLD:
The thermal emission spectrum of the Earth indicates that uncontrollable thermal runaway likely commences at an atmospheric CO2 concentration of about 433 ppmv. The present atmospheric CO2 concentration of 422 ppm is higher than at any time since the PETM. Replacing existing fossil fuel facilities with equivalent nuclear facilities is likely at least a 60 year project. In 2013 the atmospheric CO2 concentration increased at 2.66 ppm / year. This rate of increase has tripled during the last 60 years and may further increase in the near term. It is of paramount importance to stop combustion of fossil carbon to prevent further increase in the atmospheric CO2 concentration.
HOT STATE TRAPPING:
Thermal runaway will cause gradual warming of the oceans which will cause the oceans to release dissolved CO2 to the atmosphere. The resulting steady state increase in atmospheric CO2 concentration will cause the Earth to remain trapped in its "hot" state until carbonate rock and fossil fuel formation processes substantially reduce the amount of CO2 in the ocean-atmosphere pool. The geologic record shows that after previous thermal extinctions this excess CO2 removal process took about 100,000 years to operate.
The persistence time of the excess CO2 in the ocean-atmosphere pool is indicated by the right hand portion of the following graph:
CO2 Concentration decay rates
PETM
About 56 million years ago, during a time period known as the PETM (Paleocene Eocene Thermal Maximum), the atmospheric CO2 concentration raapidly increased due to combustion of all exposed organic carbon and all the polar ice melted. Mass spectrometer analysis of carbon and oxygen isotopes in and adjacent to the PETM sedimentary layer at various locations around the world give us certainty regarding the occurrence of the PETM events. The PETM duration was over 200,000 years. Fossils before, during and after the PETM indicate that during the PETM there was a global extinction of all large land animals. The PETM duration and isotope ratio data indicate that the PETM was a result of thermal runaway with hot state trapping.
PETM INITIATION:
We are quite certain that the PETM occurred as a result of the process described above. The initial event which tripped the PETM, instead of being combustion of fossil fuels by man, was likely the nearby passage of another star which caused a temporary increase in solar irradiance sufficient to trigger widespread combustion of accumulated biomass and exposed fossil fuels and hence triggered thermal runaway.
EXTINCTION:
Today we live in Goldilocks circumstances which permit the existence of large animal life on this planet. However, if mankind injects enough CO2 into the atmosphere to raise the average top of cloud temperature by 3 degrees K, Earth's planetary albedo will rapidly drop due to cloud ice crystal melting. The consequent rapid increase in net absorbed thermal power will eventually raise the ocean surface temperature by about 17.5 degrees C. The corresponding wet bulb temperature rise will cause a global extinction of large land animals.
This ocean heating process will take over a century. However, from mankind's perspective the key issue is the remaining time to when the feedback loop gain exceeds unity, after which point the thermal runaway process will be impossible to stop.
NORTH AMERICAN PUBLIC BEHAVIOR:
Given present public behavior there is no certainty that thermal runaway and a resulting global large animal species extinction can be prevented. In some countries, particularly Canada and the USA, fossil fuel interests have enormous sway with the government and most voters lack the education required to reject persistent fossil fuel producer propaganda. In both Canada and the USA the sense of entitlement to unlimited use of fossil fuels is very strong. It is analogous to the sense of entitlement on the part of the German and Japanese peoples that led to WWII.
GLOBAL PERSPECTIVE:
It is extremely important that persons in the Canadian government look at the Global Warming problem from the perspective of people in low elevation states and semi-arid countries. These people are not stupid. They know that if the present pattern of CO2 emissions continues it will only be a few years until they starve to death either due to sea water inundation or due to lack of irrigation water in the dry season.
This starvation is already happening in a number of semi-arid countries. Agricultural production is already reduced in Africa, USA and Australia for lack of irrigation water. From the perspective of the affected people the climate injustice brought on them by fossil CO2 emissions is huge. These people are more forgiving of China because China's per capita fossil CO2 emissions are far below the North American emissions and China is busy building numerous nuclear reactors to mitigate the problem. The Canadian and US governments worship the dollar in preference to human life in other countries. The recently adopted Canadian fossil carbon emissions tax is only about (1 / 10) of the level required to be effective at keeping fossil fuels in the ground.
One of the problems of our urban society is that most Canadians have lost sensitivity to the importance of dry season irrigation. A related issue is that neither the US nor the Canadian governments want the truth revealed to the North American voting pubic that current North American energy policies are causing third world starvation. The US and Canadian governmental policies are a reflection of both short term fossil fuel interests and a scientifically illiterate electorate.
The third world people on the brink of starvation have little to lose by uncontrolled migration and global conflict. North American governments blame Islamic extremists, without addressing the issue that it is North American energy policies that drive these extremists to sacrifice their own and their children's lives. It is little wonder that there is public support in emerging countries for nuclear power.
This web page last updated February 20, 2024.
| Home | Energy Physics | Nuclear Power | Electricity | Climate Change | Lighting Control | Contacts | Links |
|---|