| Home | Energy Physics | Nuclear Power | Electricity | Climate Change | Lighting Control | Contacts | Links |
|---|
This web page outlines the problem of CO2 induced ocean acidification which poses a near term threat to ocean life that forms about 15% of the world human food protein supply and is the cause of a present precipitous decline in the British Columbia wild fish population.
The main chemical reaction of concern is:
CaCO3 + H2O + CO2 = Ca(HCO3)2 = Ca++ + 2 HCO3-
To put the matter simply, as CO2 gas dissolves in ocean water it combines wih the insoluble CaCO3 ocean shell material to form water soluble Ca++ and HCO3- ions. This chemical reaction destroys sea shells and all the ocean species that rely upon them either directly for structural strength or indirectly as a food source.
The magnitude of the problem in 2019 is well summarized in the story:
Climate Change Was Killing Northwest Oyster Growers and Scientists Fought Back. This story confirms this author's personal observations regarding the shell fish and kelp populations at the British Columbia Inside Passage beaches of West Vancouver, Parksville and Comox.
The term "ocean acidification" is a fancy name for a low ocean pH resulting from an increased ocean dissolved CO2 concentration. Ocean acidification reduces the ocean (CO3)-- ion concentration which ion concentration is crucial for the survival of micro-organisms known as zooplankton that are near the bottom of the ocean food chain. Due to the limited ocean turnover in the British Columbia Inside Passage (water between the mainland and the off-shore islands) this issue of low ocean pH is now causing fish starvation in the inside passage. The observable consequences include a collapse of the herring and salmon fish populations, starvation of the resident orca population and inability of bears to put on sufficient weight to survive winter hibernation.
OCEAN FOOD CHAIN:
Ocean life as we know it can only exist at a relatively narrow range of atmospheric CO2 concentrations. The reason is simple. All ocean life requires energy. Almost all of that energy comes from phytoplankton, which are tiny green floating plants with slightly positive buoyancy. The phytoplankton contain chlorophyll and use sunlight to perform photosynthesis which converts dissolved CO2 into sugars. The energy contained in these sugars powers almost all other marine life. Hence phytoplankton are at the bottom of the ocean food chain. Note that the phytoplankton require a minimum concentration of CO2 dissolved in the water in order to function. Typically that minimum CO2 concentration results in an ocean water pH of about 8.40.
Next up the ocean food chain are micro-organisms known as zooplankton. These micro-organisms must co-exist with and eat the phytoplankton to produce a wide range of more complex organic compounds. In order to perform this function these micro-organisms have a more complex structure that requires bone or shell material which is usually based on calcium carbonate (CaCO3), also known as limestone. However, CaCO3 can only form if the dissolved CO2 concentration is below a certain maximum. Typically that maximum CO2 concentration results in an ocean water pH of about 8.00. If the pH is less than 8.00 the (CO3)-- ions preferentially form (HCO3)- ions instead of CaCO3.
Thus the micro-organisms known as zooplankton that are near the bottom of the ocean food chain can only exist if the ocean water pH is in the range 8.00 to 8.40. If the ocean water pH is less than 8.00 these micro-organisms cannot form bone and shell material. If the ocean water pH is greater than 8.40 there is not enough CO2 dissolved in the water to feed the phytoplankton which in turn are essential food for the micro-organisms known as zooplankton.
Next up the food chain are small fish such as herring. These small fish gain energy by eating the zooplankton and phytoplankton.
Further up the food chain are larger fish such as salmon. These larger fish gain energy by eating the smaller fish such as herring.
At the top of the food chain are mammals including bears, orca (killer whales) and humans. To varying degrees these mammals gain energy by eating salmon.
For many millions of years Earth's atmospheric CO2 concentration stayed within the range 180 ppmv to 300 ppmv and as a consequence the ocean pH remained in the range 8.2 to 8.4 and the (HCO2)- and (CO3)-- ion concentrations were appropriate to enable both the phytoplankton and zooplankton to exist. Over time the zooplankton micro-organisms died and their CaCO3 bone and shell components sunk to the bottom of the ocean where they gradually accumulated to form a layer of limestone. Core drilling of this layer of limestone and isotopic analysis of the drill cores reveals the geophysical history of Earth going back over one hundred million years.
THE OCEAN ACIDIFICATION PROBLEM:
The problem today is that recent large scale combustion of fossil fuels has increased the atmospheric CO2 concentration past 413.5 ppmv which causes a corresponding increase in the concentration of dissolved (HCO3)- ions in ocean water which in turn causes a corresponding decrease in the concentration of (CO3)-- ions in ocean water. This decrease in (CO3)-- ion concentration prevents micro-organisms from forming bone and shell material. Since these micro-organisms are near the bottom of the ocean food chain, loss of these micro-organisms causes collapse of the entire ocean food chain which will soon cause starvation of humans who are dependent on marine protein.
This problem of ocean acidification is particularly acute in coastal sea water where, due to a continental shelf and off-shore islands and/or long inlets, there is relatively little mixing between the CO2 absorbing near surface water and the deeper water of the major ocean. Due to ocean mixing the ocean acidification problem is presently less acute over the deep ocean but will continue to get worse during the coming decades. Presently in southern BC the herring and wild salmon populations are collapsing. Wild salmon have to swim hundreds of km through the food depleted Inside Passage while transiting to and from the rivers in which they were born and in which they spawn. Similarly resident orca are not reproducing due to lack of their principal food (salmon).
The problem of collapse of the ocean food chain due to ocean acidification by fossil CO2 was predicted by scientists decades ago, but has been overlooked by the IPCC and most other parties studying CO2 triggered climate change. However, this collapse of the ocean fishery has major near term human consequences. The time for irresponsible political and legal decisions enabling building of more fossil fuel energy infrastructure instead of building more nuclear power infrastructure has long past.
REFERENCE SLIDES BY ALEX CANNARA:
Acidification Remediation
HISTORY OF OCEAN ACIDIFICATION:
The development of knowledge about ocean acidification is outlined in the paper:
A Short History of Ocean Acidification Science.
OCEAN CHEMISTRY:
Reference: Ocean Chemistry
An authority on ocean chemistry is: Professor Andrew Dickson of the UCSD Scripps Institute of Oceanography. He has a comprehensive slide presentation titled:
Introduction to CO2 Chemistry in Sea Water
The important ocean life related chemical reactions are as follows:
Decomposition of volcanic rock under water:
1) CaSiO3(a portion of volcanic rock) + H2O > Ca(OH)2 + SiO2 (sand)
2) Ca(OH)2 > Ca++ + 2(OH)-
(The (OH)- ions make sea water slightly basic)
Dissolving a small amount of CO2 from the atmosphere into ocean water causes the reaction:
CO2 + H2O = 2 H+ + (CO3)--
The low resulting small H+ ion concentration is cancelled by the larger (OH)- ion concentration via the reaction:
2 H+ + 2 (OH)- = 2 H2O
so the sea water remains basic but at a slightly lower pH.
Recall that a pH of 7.0 is a neutral solution, a larger pH is basic, a smaller pH is acidic. Before combustion of fossil fuels the ocean pH was about 8.4, which is basic. Increasing the atmospheric CO2 concentration causes the ocean pH to drop below 8.0 which is still basic but is more acidic than at a pH of 8.4. Hence this process is known as ocean acidification. At pH values below 8.0 micro-organisms dependent on CaCO3 for formation of bone and shell material cannot survive.
Increasing the atmospheric CO2 concentration dissolves CaCO3 and sharply reduces the ocean (CO3)-- ion concentration by converting (CO3)-- ions into (HCO3)- ions via the reaction:
H2O + CO2 + (CO3)-- > 2 (HCO3)-
(Since the equilibrium concentration of (CO3)-- ions is much less than the equilibrium concentration of (HCO3)- ions a small fractional change in (HCO3)- ion concentration causes a large fractional change in the (CO3)-- ion concentration.)
Formation of CaCO3 by tiny marine organisms:
Ca++ + (CO3)-- > CaCO3
(This is the chemical reaction used by marine species to form bone and shell material)
Ocean heating:
2 (HCO3)- > H2O + CO2 + (CO3)--
(Water temperatures that are high enough to reverse the ocean acidification process are over 45 degrees C and are incompatible with most marine life)
As long as the atmospheric CO2 concentration is low the (CO3)-- ion concentration in ocean water is sufficiently high that CaCO3 can form in the bones and shells of simple marine organisms.
However, reactions #1 and #2 above are very slow which limits the rate at which Ca++ and (CO3)-- ions can form. When the atmospheric CO2 concentration is higher than normal the rate of (HCO3)- ion formation exceeds the rate of (CO3)-- ion formation. Under these circumstances the (HCO3)- ion concentration in the ocean water rises and the (CO3)-- ion concentration drops preventing CaCO3 formation. Thus one of the near term consequences of continued combustion of fossil fuels is extinction of the micro-organisms that rely on CaCO3 for production of bone or shell material. Loss of these micro-organisms causes the entire ocean food chain to collapse as indicated by loss of herring, salmon, orcas and bears.
OBSERVATION COMPLICATIONS:
Observations of ocean acidification via the distribution of CaCO3 on the ocean floor and via dissolved CO2 concentration are complicated by the natural flux of CO2 and CaCO3 through the ocean. Atmospheric CO2 preferentially dissolves in the ocean in cold near polar waters and comes out of solution in warm tropical waters. Thus, even with no combustion of fossil fuels the concentration of dissolved CO2 and the rate of deposition of CaCO3 on the ocean floor varies widely with latitude and water temperature. The practical way to observe and quantify ocean acidification is via precise measurements of ocean water pH using sophisticated chemical instrumentation. However, due to the complexity of the required instrumentation there are limited available ocean pH data sets.
pH EXTINCTION LEVEL:
Japan has long recognized the importance of ocean pH to fish and has carefully monitored Pacific Ocean pH at various points since about 1980. After the trend of decreasing ocean pH became obvious the Japanese ocean pH monitoring program was expanded in 1995. Japanese ocean pH data alerted the world to the link between atmospheric CO2 concentration and ocean pH. This issue, now called Ocean Acidification, was the subject of a world conference in Monaco in October 2008. That conference resulted in the Monaco Declaration On Ocean Acidification dated January 30, 2009.
Prior to the year 2008, the Scripps Institute of Oceanogrpahy confirmed by experiment that an ocean pH below 8.0 would prevent bone and shell formation in many marine organisms. At lower ocean water pH values the shell material dissolves. Prior to 2008 the bulk ocean pH was still sufficiently high that ocean acidification was not an immediate threat.
MEASUREMENTS:
While the ocean volume is very large the amount of CO2 dissolved in the ocean due to combustion of fossil fuels is also large and the fraction of CO2 in ocean water necessary to significantly lower the ocean pH is relatively small. The rate at which CO2 dissolved in the ocean is removed by formation of insoluble CaCO3 is orders of magnitude smaller than is the present rate of CO2 absorption by the ocean. Hence there is an increasing accumulation of (HCO3)- ions in ocean water. The mass of CO2 dissolved in ocean water via the reaction:
CO2 + H2O > H+ + (HCO3)-
is comparable to and follows the mass of CO2 in the atmosphere, which mass is constantly increasing. The increase in ocean (HCO3)- ion concentration is revealed via precise measurement of the pH of ocean water.
Note that historically the ocean pH was about pH = 8.30 and that an ocean food chain collapse occurs at a pH values below pH = 8.00.
To demonstrate the extent of the bulk ocean acidification problem it is necessary to plot ocean pH as a function of time over a period of decades. The following Japanese bulk ocean pH data was provided to this author by Dr. Alexander Cannara of Menlo Park, California. His contact information is:
Email: cannara@sbcglobal.net, Telephone: 650-400-3071
Cannara Supplied Data:
OCEAN pH DATA
or
Japanese Pacific Ocean pH data
The following ocean water pH data was provided to this author by Mr. Alex Rhodes, of Victoria, British Columbia, formerly a founder and principal of the whale watching company Seacoast Expeditions:
Ocean water pH data from an off-shore buoy by Washington State
and
US Ocean Water pH Data
and
Ocean Networks Canada
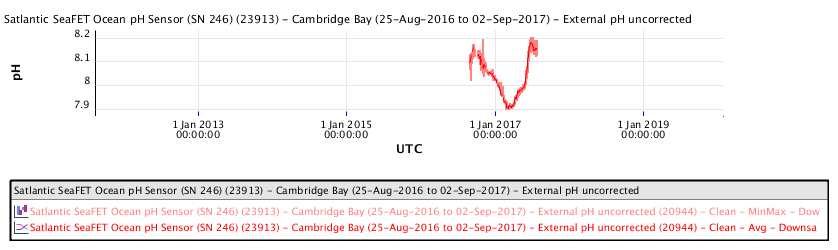
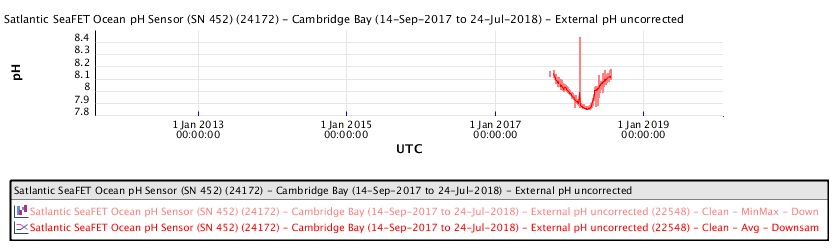
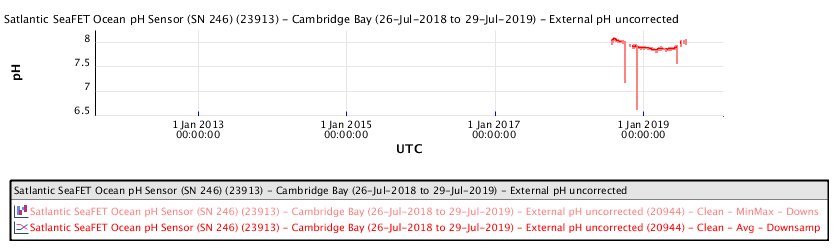
Ocean water pH measurements made at Cambridge Bay, Nunavut in the high Arctic, during the period 2016 to 2019, show the following ocean water pH as a function of time:



US ocean water pH measurements made in 2014 near Prince Rupert, BC which is adjacent to the southern tip of the Alaska panhandle, show the following pH as a function of time:
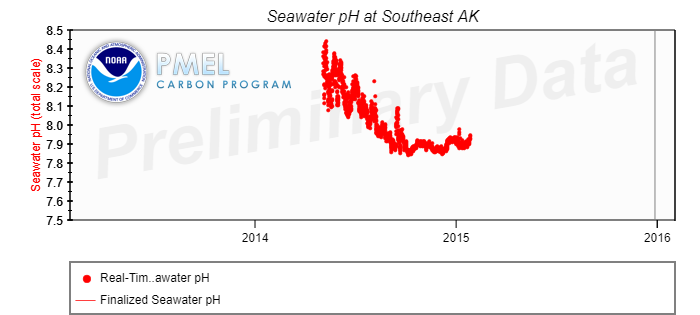
The above data shows that near Prince Rupert in the later part of 2014 the ocean water pH decreased to the point of causing a local ocean food chain collapse.
Available ocean water pH measurements in the Salish Sea between Vancouver and Victoria show the following pH measurements as a function of time:
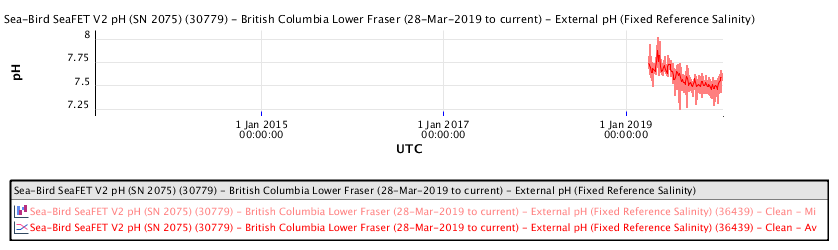
The Marine Technology Centre is near the Vancouver Island Swartz Bay ferry terminal, north of Victoria.
The recent pH data for the Salish Sea consistently indicates a pH of consistently less than 8.00 corresponding to a local ocean food chain collapse. This data explains the precipitous decline in the herring and salmon population and the failure of resident orcas to successfully reproduce during recent years.
The US data from the buoy at La Push off the coast of Washington State shows a trend of gradually decreasing pH in the deep ocean off the coast of Washington State.
If present trends continue the Japanese data provided by Dr.Alexander Cannara and the data from the ocean buoy at La Push (Washington State) show that most major ocean fish species will be extinct before the year 2050. This extinction will be the result of acidification of ocean water caused by the increased atmospheric CO2 concentration. The relatively low ocean turnover in the BC Inside Passage, including the Salish Sea, exacerbates the ocean acidification problem. In the bulk Pacific Ocean part of the absorbed CO2 diffuses into the deep ocean. In the BC Inside Passage the average depth is of the order of (1 / 10) the depth of the main Pacific Ocean, so the absorbed CO2 is more concentrated, making the pH significantly lower than in the bulk ocean.
Videos:
A 5 minute summary of Ocean Acidification presented by Dr.Alexander Cannara in 2015
A 56 minute lecture on Ocean Acidification and related chemistry presented in January 2009 by Dr. Andrew Dickson, a Scripps Institute marine chemist.
This video contains scanning electron microscope photos which demonstrate that at an ocean pH of less than 8.00 many marine organisms can no longer maintain bone and shell material. Note: The change from ocean pH = 8.15 to ocean pH = 8.05 represents a 25% increase in ocean acidity due to CO2 absorbed since 1980.
SUMMARY:
Ocean acidification is a major problem because the micro-organisms directly impacted by a decrease in ocean (CO3)-- ion concentration are near the bottom of the ocean food chain. These micro-organisms are the main source of food for small fish such as herring which in turn are the main source of food for larger fish such as salmon which in turn are the main source of food for large marine mammals such as orcas and are an important source of food for land animals such as bears and humans. Bear excrement is an important source of micro-nutrients in many forests.
As the atmospheric CO2 concentration rises the ocean (CO3)-- ion concentration rapidly falls which causes the micro-organisms known as zooplankton to die, which leads to death by starvation of species further up the food chain including herring, salmon, marine mammals, bears and ultimately humans. Ocean pH measurements indicate that there has been ocean starvation in the British Columbia Inside Passage since about 2014. If present trends continue Japanese data indicates that most major fish species in the bulk ocean will be extinct by the year 2050.
There is presently a rapid decline in wild fish stocks on both the east and west coasts of Canada. On the west coast ocean food chain starvation has caused a precipitous decline in the herring and salmon populations which has directly impacted the populations of large mammals such as orcas and bears. There is no practical remedy for this ocean acidification problem other than leaving fossil fuels in the ground.
Chemically correcting the ocean pH, even just in Canadian inshore waters, would require a fleet of nuclear reactors and many cubic km of mined material. The unwillingness of natioonal governments to face the reality and consequences of rising atmospheric CO2 concentration is rapidly leading to a human disaster. Much of the human population of the island nations of south east Asia relies on sea food protein. As sea food protein supplies collapse there will be forced migration of about a billion people, many of whom will attempt to seek refuge North America.
In Canada the decision by the Canadian supreme court on January 16, 2020 to allow tripling of the capacity of the Transmountain Pipeline, against the will of the elected government of British Columbia, which sought to protect its environment, will quickly cause irreversible destruction of the fishing industry in British Columbia and the US Pacific North West, even without a major maritime bitumen spill. The existing Alberta oil sand production, if continued, will cause a further cumulative reduction in bulk ocean pH which, over about two decades, will drive most major fish species into extinction. The oceans are further threatened by the increase in tar sand production capacity enabled by the Transmountain Pipeline capacity expansion.
Due to the limited ocean turnover in the BC Inside Passage, the pH in the Inside Passage, including the Salish Sea, is significantly lower than in the bulk ocean. By enabling expansion of tar sand oil extraction the courts and the Canadian federal government are permanently sacrificing the BC fishing industry and all it supports in order to realize a few years of limited oil profits. This is an extremely foolish decision that Canadians will likely regret for centuries to come.
It is equally foolish for Indian bands to take an ownership position in the Trans-Mountain Pipeline. By so doing these Indian bands are enabling their own economic demise. How can they claim compensation for loss of the herring and salmon fishery and fish dependent marine mammals when they are owners of the pipeline that in the future will be the main Canadian enabler of ocean acidification?
Some Indian bands have fishing rights protected by treaty. Absent Indian ownership of the Trans-Mountain Pipeline these Indian bands will likely have a strong legal case for long term financial compensation due to federal government enabled destruction of the BC herring and salmon fisheries by the Alberta fossil fuel industry.
The prudent thing for all parties to do right now is to cancel the Trans-Mountain Pipeline capacity expansion, close tar sand oil production and obtain the required energy from CO2 emission free nuclear power. Unfortunately short term greed is overwhelming long term prudence.
OCEAN CHEMISTRY:
Volcanic rock very slowly corrodes:
Ca2SiO4 (volcanic rock) + 2 H2O = 2 Ca++ + 4 (OH)- + SiO2 (sand)
This is a rate limiting reaction.
Near the ocean surface CO2 gas dissolves in cold water:
2 CO2 (gas) + 2 H2O (liquid) = 2 H+ + 2 (HCO3)-
At the near surface mixing point where the ocean is warmer:
2 Ca++ + 4 (OH)- + 2 H+ + 2 (HCO3)- = 2 CaCO3 + 4 H2O
Marine creatures form CaCO3 shells which on the death of the creature sink to and accumulate on the ocean floor.
Raising the atmospheric CO2 concentration makes the ocean more acidic (lower pH) and tends to dissolve CaCO3 sea shell material.
CO3--(slightly soluble ion) + CO2 + H2O = 2 (HCO3)- (highly soluble ion)
OCEAN ACIDIFICATION ARTICLE AND VIDEO REFERENCES PROVIDED BY ALEX CANNARA:
Our Daily Planet
Seattle times
Phys.Org
Physics World
University of Technology, Sydney
California Natural Resources
Hakai Magasine
Smithsonian
Facebook
ABC.Net.Au
Motherboard Tech By Vice
Carbon Brief
You Tube
Nature
PLOS ONE
Facebook
Investigate West
Science Daily
ScienceDaily
CBC
The Guardian
Sea-Bird Scientific
A Short History of Ocean Acidification Science
This web page last updated December 12, 2021.
| Home | Energy Physics | Nuclear Power | Electricity | Climate Change | Lighting Control | Contacts | Links |
|---|